1.0 Science Overview
1.1 General Description of Stratospheric Ozone
1.1.1 What is ozone?
Ozone is an atmospheric gas which screens a harmful form of solar radiation, known as ultraviolet radiation, from reaching the Earth. About 90% of the ozone in our atmosphere is contained in the stratosphere (the region from about 30,000 feet to 180,000 feet above the Earth's surface between the tropopause and stratopause). Ozone concentrations are greatest between about 50,000 and 100,000 feet, but these ozone concentrations are very small, typically comprising only a few molecules per million molecules of air (air is composed of about 78% nitrogen molecules and 21% oxygen molecules).
Scientific studies of ozone are important because we do not yet understand how the ozone layer may be changing, and how that may affect the habitability of the planet. Without protective upper-level ozone, there would be no life on Earth. Exposure to ultraviolet radiation is a primary cause of skin cancer, and will be exacerbated as ozone levels diminish. In contrast, low-level or tropospheric ozone is a greenhouse gas. Stratospheric ozone losses are believed to cool the Earth's surface, while ozone increases in the lower atmosphere will act to warm the Earth's surface.
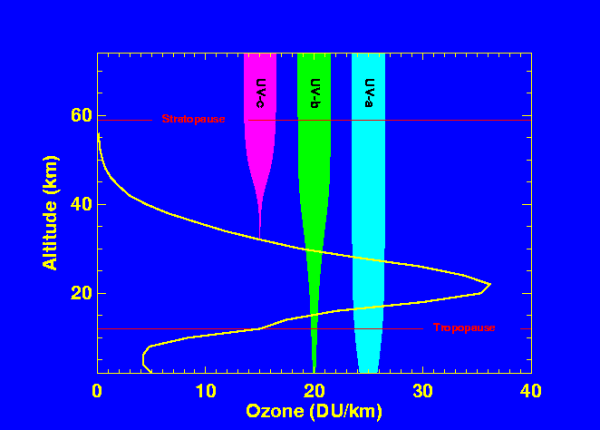
Figure 1 shows a typical profile of ozone density versus altitude (yellow line) in the midlatitudes of the Northern Hemisphere (units=Dobson Units/kilometer). The stratosphere lies between the tropopause and stratopause (marked in red). Superimposed on the figure are plots of UV radiation as a function of altitude for UVa (320-400 nm, cyan), UVb (280-320 nm, green), and UVc (200-280 nm, magenta). The width of the bar indicates the amount of energy as a function of altitude. The UVc energy decreases dramatically as ozone increases because of the strong absorption in the 200-280 nm wavelength band. The UVb is also strongly absorbed, with a small fraction reaching the surface. The UVa is only weakly absorbed by ozone, with some scattering of radiation near the surface.
1.1.2 UV Effects and Ozone
Absorption of harmful solar ultraviolet radiation is critical for our well being, since ultraviolet light can break the bonds of DNA molecules (the molecular carriers of our genetic coding), and thereby damage cells. While most plants and animals are able to either repair or destroy their damaged cells, on occasion, these damaged DNA molecules are not repaired or eliminated, and can replicate, leading to dangerous forms of skin cancer in humans (basal, squamous, and melanoma). Fortunately, ozone strongly absorbs UV. Note how the magenta bar in Figure 1 above quickly diminishes as the solar energy penetrates to levels where ozone is found. UVb is also strongly absorbed, but some UVb can penetrate to the surface. If ozone were reduced by 1%, there would be about a 2% increase of surface UVb. Diminished levels of ozone lead to increases of UVb and UVa radiation at the surface with a consequent adverse biological response.
1.1.3 Ozone Production and Loss
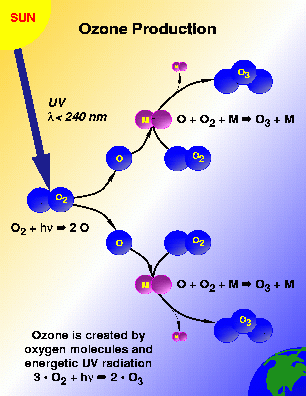
Ozone is produced by the intense ultraviolet radiation in the upper stratosphere at wavelengths generally less than 240 nm. This radiation breaks apart an oxygen molecule into oxygen atoms and these atoms in turn react with other oxygen molecules to form ozone molecules. This ozone production process is shown schematically in Figure 2.
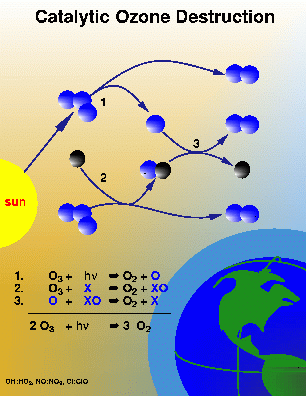
Ozone is destroyed when it reacts with one of a variety of chemicals in the stratosphere such as chlorine, nitrogen, bromine or hydrogen. This process occurs in a three-step catalytic process. The first ozone molecule is photolyzed to form an O atom and the first oxygen molecule. Second, the catalyst reacts with another ozone molecule to form XO and the second oxygen molecule. Finally, the XO molecule reacts with the O atom to form a third oxygen molecule, and to reform the original catalyst. The catalyst converts two ozone molecules into 3 oxygen molecules without being affected itself. A typical chlorine atom can destroy thousands of ozone molecules in this fashion. Figure 3 portrays the destruction of ozone in a homogeneous gas phase reaction.
The catalyst (X, the black molecule) in Figure 3 represents a chlorine, bromine, hydrogen, or nitrogen atom. Such reactions occur naturally, and the global ozone levels are approximately balanced between the solar production and chemical losses from these processes. Increasing the levels of chlorine and other pollutants in the stratosphere could increase the chemical loss process, and subsequently decrease the ozone levels.
Substantial polar ozone loss requires three key ingredients. First, there must be significant levels of inorganic chlorine for conversion into radical forms. This condition has been true since the early 1980s as a result of pollution of the stratosphere by chlorofluorocarbons. Second, temperatures must be cold enough (below about 195 K) for PSCs to form. This always happens in the Arctic by early December. Finally, the cold temperatures must persist late into the winter and early spring when the sun is fully risen over the Arctic.
1.1.4 Polar Ozone Science
The lower stratosphere during the Arctic winter is a strange environment for both chemistry and meteorology. During the winter, Arctic temperatures at about 20 km can fall below -120 F (-83 C). In the Antarctic, the temperatures are even colder, occasionally falling below -136 F. Although the stratosphere is very dry, these extremely cold temperatures cause clouds to form. These polar stratospheric clouds (PSCs) enable very interesting chemical reactions (known as heterogeneous chemical processes) that accelerate ozone loss. In fact, these heterogeneous reactions cause the Antarctic ozone "hole." Scientists are increasingly worried that cooler temperature in the Arctic may lead to increased formation of PSCs, and thereby massive ozone losses. The ability to predict these losses requires detailed understanding of the chemistry, dynamics, and radiative properties of the Arctic stratosphere.
1.1.4.1 Antarctic and Arctic Ozone Levels
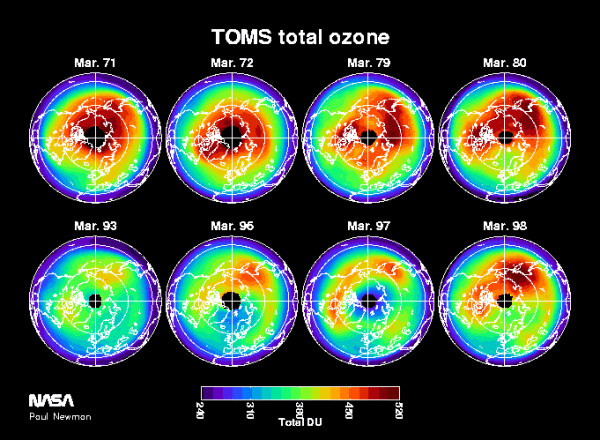
The Antarctic ozone "hole" is a region of extreme ozone loss that has been appearing annually since the 1970s. Ozone amounts over Antarctica drops dramatically (up to 50%) in the course of a few weeks during the August-September period. The ozone hole results from the increased amounts of chlorine and bromine in the stratosphere, combined with the peculiar meteorology and extreme cold of the Southern Hemisphere winter. Figure 4 shows October averages of satellite data for a series of early years, prior to the appearance of the ozone hole (top panels), and the last few years (bottom panels). These averages are determined from satellite column ozone as measured by Nimbus-4 BUV (1970, 72, 72), Nimbus-7 (1979), Meteor-3 (1994), SBUV (1996), and EP-TOMS (1997, 98) satellites. The color scale on the bottom indicates high ozone levels in red, and low ozone in the blues and purples.
The growth of chlorine and bromine levels in the stratosphere has produced very large losses of ozone over the polar regions, producing this Antarctic ozone hole. During the 1990s, ozone levels are less than half of what was observed during the 1960s and 70s. Losses of a similar magnitude have been observed over the Arctic for a number of years.
Figure 5 shows polar averaged Arctic ozone levels. Because of the different climates of the Arctic and Antarctic, the Arctic ozone levels are naturally higher, yet both polar regions have shown reduced ozone over the last decade. The March averages of total ozone as measured by Nimbus-4 BUV (1971, 72), Nimbus-7 (1979, 90, 93), NOAA-14 SBUV-2 (1996), and EP-TOMS (1997) satellites. Note the differences in color scales between Figures 4 and 5; northern ozone levels are naturally much higher than southern levels.
1.1.4.2 Polar Stratospheric Clouds
The extremely low ozone which occurred in the Arctic during the 1990's also coincided with colder than normal temperatures. The cold temperatures led to an increase in the occurrence of polar stratospheric clouds (PSCs). The PSCs led to increased ozone loss via the heterogeneous reactions that occur on the cloud surfaces. This is discussed in the following sections.
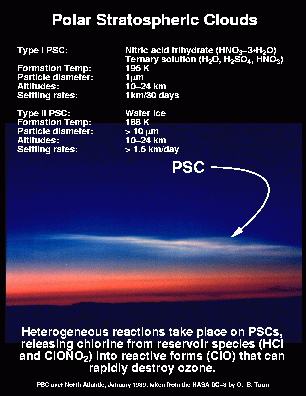
As the stratosphere cools to very cold temperatures over the Antarctic during the southern winter, PSCs form. Figure 6 shows a photograph of a PSC taken by Brian Toon near Iceland during the AASE I campaign in 1989. PSCs are particularly prevalent in the Northern Scandinavia region during the winter period. Kiruna, Sweden is an optimal site (see section 2.3) for sampling PSCs, since the stratosphere above Kiruna is normally quite cold, and the local meteorology (mountain forced waves) creates even colder conditions, leading to PSC formation.
(Photo courtesy of O. B. Toon).
PSCs are principally responsible for the observed ozone losses in the polar region. These PSCs convert inorganic chlorine from reservoir species (HCl and ClONO2) to free radical form as in the Antarctic [see Brune et al., 1990; Toohey et al., 1993]. This conversion occurs via heterogeneous reactions on the surfaces of stratospheric particles and aerosols. Direct in-situ observations of PSCs are rather rare, having only occurred during a few flights of the ER-2 during the SOLVE-I, AAOE, AASE-I, and ASHOE/MAESA campaigns.
1.1.4.3 Dynamics and Transport
The polar lower stratosphere becomes extremely cold during winter, since the polar night jet acts to contain the polar air during mid-winter. This polar air mass is known as the polar vortex. As viewed from space above the North Pole, this appears as a counterclockwise swirl of air. Because of this containment, this polar vortex air has a distinctive chemical composition as compared to air in the mid-latitudes. This containment makes the polar vortex a "containment vessel" for the chemical ozone loss that takes place during the winter and spring.
The air found in the polar vortex principally originates from higher stratospheric altitudes. The air generally moves poleward and downward over the course of the winter period. Since ozone values increase with altitude, this poleward and downward motion increases the column ozone amount. Therefore, the average transport of air toward the pole during the winter will act to increase the column ozone over the course of winter.
1.1.5 Ozone and Public Policy
Reported ozone losses and predictions of ozone loss have heightened our concern with the effects of increased UV levels. Such concerns have led to international treaties such as the Montreal Protocol, which have regulated the production of certain gases which can harm the ozone layer. Further research and measurements have begun to provide insight into how other atmospheric processes (such as greenhouse warming) can effect ozone. This further research in necessary to refine our understanding of the stratosphere, leading to well based policy decisions.
1.2 General Description of SOLVE-II Mission
The second SAGE III Ozone Loss and Validation Experiment (SOLVE-II) is an atmospheric science measurement campaign in the Arctic high-latitude region planned for January-April 2003 that will focus on acquiring correlative data needed to validate measurements from the Meteor-3M/Stratospheric Aerosol and Gas Experiment (SAGE) III satellite mission. This includes measurement of ozone, aerosols, and trace gases from NASA's DC-8 aircraft along with balloons, rockets, and sondes. The DC-8 will fly a series of flights in and around the polar vortex on a variety of flight trajectories for temporal and spatial coincident data acquisition using both remote sensing and in situ detection measurements. The field campaign will also acquire correlative measurement with the atmospheric chemistry instruments onboard the POAM and ENVISAT satellite missions to enhance comparison and science activities utilizing these data sets.
The SOLVE-II mission will include profile measurements of ozone, water vapor, nitrogen dioxide, temperature, pressure, and aerosol size distribution from small balloons. These measurements will provide linkage to previous satellite validation activities and will serve as a valuable cross-reference to other long-term records. It is envisioned that such balloon payloads will be launched from the Esrange facility near Kiruna, Sweden, which will be at approximately the same latitude of measurement for SAGE III sunset occultation events during the campaign. It is anticipated that approximately 6-8 balloon launches will be conducted during the campaign. It is also possible that one or two heavy lift balloons carrying remote sensing instrumentation will be launched during the January-March, 2002 timeframe.
This campaign will be conducted in close collaboration with the VINTERSOL (Validation of International Satellites and study of Ozone Loss) campaign sponsored by European Commission. More than 350 scientists from the United States, the European Union, Canada, Iceland, Japan, Norway, Poland, Russia, and Switzerland will participate in this joint effort. SOLVE-II is co-sponsored by the Upper Atmosphere Research Program, the Radiation Sciences Program, the Atmospheric Chemistry Modeling and Analysis Program, and Earth Observing System of NASA's Earth Science Enterprise.
1.3 SAGE III Validation
A major objective of SOLVE II is the validation of the SAGE-III instrument, which was launched on the Russian Meteor 3M spacecraft on December 10, 2001. SAGE III is the latest in a family of solar occultation satellite instruments designed to monitor distributions of stratospheric and upper tropospheric aerosol, ozone, water vapor, and other important chemically active trace species with very high vertical resolution of approximately 0.5 km. Measurements from SAGE III will extend the approximately 17 year data record collected by SAGE II (1984-present) and provide a valuable data resource that will enable an examination of long-term climate variations and trend analysis.
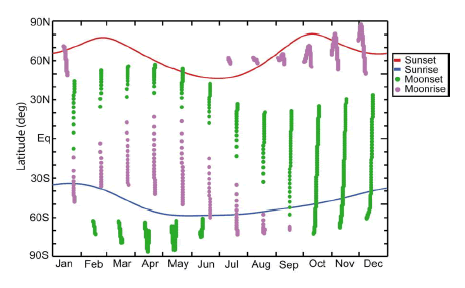
For the Meteor 3M/SAGE III mission, solar occultation measurements will occur mostly at high latitudes in the Northern Hemisphere and mid latitudes in the Southern Hemisphere. This coverage will allow measurements to be made within the Arctic polar vortex where polar stratospheric clouds form and rapid ozone loss occurs locally in winter and early spring. Greater latitudinal coverage is available from lunar occultation events. Figure 7 shows the expected latitudinal coverage of SAGE III measurement locations throughout the year.
Validation of SAGE III science data requires airborne and balloon correlative measurements that are in close temporal and spatial coincidence to reduce uncertainties in representative sampling. Added correlative measurements are also needed along the line-of-sight between the satellite and the sun (or moon) to assess the impact of constituent inhomogeneity on the retrieval algorithm. This is especially true in the presence of polar stratospheric clouds or cirrus. The measurement requirements specified below are discussed further in the SAGE III Validation Plan 2000.
1.4 SOLVE-II Science Objectives
The SOLVE-II campaign is designed to pursue five basic science objectives. These objectives are:
- Understanding polar ozone loss rate in early to mid-winter. Observations during the first SOLVE campaign (see section 1.6) showed a larger than expected loss of ozone during the January-February period. During SOLVE-II DC-8 flights and balloon observations will be designed to again measure ozone losses.
- Improving our understanding of polar stratospheric clouds (PSCs). Observations during the first SOLVE campaign revealed the unexpected presence of very tenuous PSCs composed of large particles in the high Arctic region. These large particles were shown to be composed of nitric acid and water and contribute to ozone depletion. However, the formation of these large particles is not understood.
- Improving our understanding of the chemistry of ozone loss. Arctic ozone is destroyed by chlorine and bromine gases that primarily come from human-produced compounds. Measurements of these compounds during the first SOLVE campaign showed that the observed decrease of ozone was in reasonable agreement with the observed levels of chlorine and bromine. During the SOLVE-II campaign, we will also be testing our observed ozone losses against these chlorine and bromine levels.
- Meteorological impacts on polar ozone levels. Over the course of the winter, ozone typically increases in the polar region, as ozone rich air in the mid and high altitudes is carried poleward by the winds. This motion also acts to warm the polar region. Because meteorological conditions vary widely between winters, this transport of ozone-rich air must be carefully identified during a winter campaign.
- SAGE III instrument validation. Ozone, aerosol, water vapor, and nitrogen dioxide measurements from the DC-8 will be compared to SAGE III measurements to demonstrate the quality of satellite observations. The satellite observations are key components of the international effort to determine the current state of the ozone layer, and to determine how it will evolve in the future.
1.5 Measurement Priorities
Based on the five science objectives described above, the SOLVE-II measurement priorities (and selection of appropriate measurement instruments) were developed as follows:
Ozone Concentration
SAGE III will measure ozone concentration from cloud top to 85 km with an accuracy of about 6% at the peak and a vertical resolution of 0.5 km. By contrast, this mission will focus on obtaining correlative ozone measurements in the upper troposphere and lower to mid-stratosphere. Accurate knowledge of ozone trends in this region near the tropopause is critical for assessing radiative forcing. Historically, this is the same altitude regime in which SAGE II has had its greatest uncertainties in ozone trend assessments.
To address the vertical extent of the SAGE III and other ozone measurement variability over the slant path (line-of-sight) of approximately 200 km for a given altitude, we will include zenith and nadir viewing lidar measurements of ozone concentrations on the DC-8 instrument payload. Lidar measurements provide high precision, but have lower accuracy; thus, an in situ ozone concentration measurement will also be included. Lidar profile measurements will extend to an altitude of at least 10 km above flight altitude. In situ observations will also cover the operational altitude range of the aircraft.
Aerosols
SAGE III will measure aerosol extinction at seven wavelengths ranging from 385 to 1550 nm in the troposphere and stratosphere. Validation of these measurements, as well as derived products such as aerosol surface area, will be based on data comparisons between instruments.
Lidar aerosol and cloud measurements
Vertical lidar backscatter measurements provide an excellent method for examining the vertical and horizontal variability of aerosol and optically-thin clouds along the SAGE slant path. Because the aerosol lidar retrieval is strongly affected by the normalization procedure, measurements should reach heights above 30 km, where Rayleigh scattering dominates the backscatter return. Measurements must have 0.5 km vertical resolution or better. Depolarization measurements are further required to discriminate between spherical and non-spherical particles.
In situ particle samplers
The multi-wavelength SAGE III aerosol measurements provide estimates of aerosol surface area. Well-calibrated instruments are needed to validate these estimates over aerosol particle size distributions in the sub-micron size range. Aerosol composition in the stratosphere and upper troposphere is needed to examine variability of the backscatter-to-extinction conversion factor. Instruments should be able to measure particle optical surface area to better than a factor of two.
Solar radiometer
Radiometers can provide slant-path optical depth measurements at many of the same wavelengths measured by SAGE III. Such measurements can further be differentiated during aircraft ascents or descents to yield vertical extinction profiles for intercomparison. These data will also enable profiles of aerosol extinction to be compared with those derived from in situ particle samplers or inferred from lidar backscatter observations.
Water vapor
In situ water vapor measurements will be needed with an accuracy of approximately 10% for the upper troposphere and less than 25% for the stratosphere. Lidar profile measurements will also be needed to examine its variability in the troposphere along the SAGE III slant path. Measurements are also desired in the stratosphere, where variations near PSCs could provide insight on cloud formation. Such profile measurements should extend at least 10 km above and 5 km below flight altitude in clear sky conditions.
Temperature, Pressure and Altitude Registry
Temperature profiles also will be used to help interpret PSC measurements and to calculate the altitude of the tropopause. Profile measurements are needed from a point several kilometers below the aircraft to a height of about 35 km.
The accurate determination of the transmission profile is fundamental to the retrieval of all species. For SAGE I, altitude registration errors of about 200 to 400 m resulted in biases in ozone mixing ratio and other species. For SAGE III, altitude registration with an uncertainty of < 100 m is desired. Validation may be achieved by measuring the altitude of the top of an optically thick cloud that lies along the slant path. It may also be achieved by matching profiles of aerosol, ozone, and water vapor, especially near the tropopause where strong vertical gradients exist. A combination of upward and downward viewing lidar profiles acquired along aircraft survey and staircase flights should provide this information.
Nitrogen Dioxide and Chlorine Dioxide
Correlative observations of NO2 and OClO are needed to compare with SAGE III measurement profiles. Nitrogen dioxide will be observed during both solar and lunar occultation events and are needed over the altitude range of about 20 km to 35 km. OClO will only be observed during lunar occultation events.
In situ Conditions
DC-8 facility measurements of time, position (latitude, longitude, and both radar and pressure altitude), attitude angles, and in situ temperature will be made to time correlate data sets obtained during mission operations.
1.6 SOLVE I Results
Between November 1999 and April 2000, two major field experiments, the first Stratospheric Aerosol and Gas Experiment (SAGE) III Ozone Loss and Validation Experiment (SOLVE) and the third European Stratospheric Experiment on Ozone (THESEO 2000), collaborated to form the largest field campaign yet undertaken to study Arctic ozone loss. This international campaign involved more than 500 scientists from over 20 countries. These scientists made measurements across the high and middle latitudes of the Northern Hemisphere.
The main scientific aims of SOLVE/THESEO 2000 were to study the processes leading to ozone loss in the Arctic vortex and the effect on ozone amounts over northern midlatitudes. The campaign included satellites, research balloons, six aircraft, ground stations, and scores of ozonesondes. Campaign activities were principally conducted in three intensive measurement phases centered on early December 1999, late January 2000, and early March 2000. Observations made during the campaign showed that temperatures were below normal in the polar lower stratosphere over the course of the 1999-2000 winter. Because of these low temperatures, extensive polar stratospheric clouds (PSC) formed across the Arctic. Large particles containing nitric acid trihydrate were observed for the first time, showing that denitrification can occur without the formation of ice particles. Heterogeneous chemical reactions on the surfaces of the PSC particles produced high levels of reactive chlorine within the polar vortex by early January. This reactive chlorine catalytically destroyed about 60% of the ozone in a layer near 20 km between late January and mid-March 2000, with good agreement being found between a number of empirical and modeling studies. The measurements made during SOLVE/THESEO 2000 have improved our understanding of key photochemical parameters and the evolution of ozone-destroying forms of chlorine.
2.0 Science Implementation
2.1 Instruments
Once the science objectives and measurement priorities of the SOLVE campaign were identified, a NASA Research announcement (NRA-02-OES-02) was issued to select appropriate investigations and instruments. The selected aircraft instruments are mapped to these priorities in Table 1. Detailed information on the instruments which comprise the SOLVE-II mission can be found here.
Desired |
Priority |
Measurement |
Instruments |
Principal |
|---|---|---|---|---|
O3 Concentration |
1 |
lidar, |
DIAL |
E. Browell/LaRC |
Aerosol Extinction |
1 |
lidar, |
FCAS/NMAS |
M. Reeves/U. Denver |
H2O Concentration |
1 |
lidar, |
DACOM/DLH |
G. Diskin/LaRC |
Temperature/Pressure |
1 |
lidar, |
MTP |
M. Mahoney/JPL |
NO2 |
2 |
solar radiometer, |
DIAS |
R. Shetter/NCAR |
OClO |
2 |
inferred from microwave radiometer |
||
Solar Imager |
2 |
CCD imager |
DIAS |
R. Shetter/NCAR |
Long-lived Tracers |
2 |
in situ techniques |
DACOM/DLH |
G. Diskin/LaRC |
HNO3 |
2 |
Mass spectrometry, |
PANTHER |
J. Elkins/NOAA |
ClO, BrO |
2 |
Microwave radiometer |
||
Reservoir Compounds |
2 |
Remote-sensing Techniques |
Table 1: Measurement requirements and candidate instrumentation for the DC-8
2.2 Platforms
2.2.1 Aircraft
The DC-8 aircraft, based at NASA's Dryden Flight Research Center, is the primary measurement platform for the SOLVE-II mission. The DC-8 provides a unique airborne science platform for a wide variety of experiments, collecting data in support of scientific projects which serves the world scientific community. Included in this community are NASA, federal, state, academic, and foreign investigators. Data gathered at flight altitude and by remote sensing from the DC-8 have been used in many studies including: archaeology, ecology, geology, hydrology, meteorology, oceanography, volcanology, atmospheric chemistry, soil science, biology, and other earth science disciplines.
The DC-8 aircraft used for this campaign is a medium altitude, moderate to high speed aircraft flying up to 41,000 feet above sea level between 425 and 490 knots True Air Speed (TAS). The DC-8 is capable of precise flight line navigation by means of an integrated inertial and GPS navigation systems from which line guidance is provided to the pilots. Further information on the capabilities of the aircraft can be found here.
In addition, VINTERSOL scientists will concurrently take measurements of the stratosphere using a large suite of instruments aboard the European high-flying M55 Geophysica aircraft, and the German DLR Falcon. A temperature profiling instrument from NASA's Jet Propulsion Laboratory will also fly on the M55 Geophysica. These aircraft will also be based in Kiruna, Sweden.
2.2.2 Balloons and Rockets
Balloon-borne in situ or remotely sensed observations of O3, NO2, and H2O will provide valuable profile data at latitudes near coincident with SAGE III sunset occultation measurements of these same species. In situ measurements of aerosol size distribution and surface area will similarly provide validation for SAGE III estimates of these parameters. Concurrent temperature and pressure measurements are also required to determine altitude and aid in scientific analysis. Additional measurements HNO3, BrO, ClO, CO, CO2, CH4, CFC-11, CFC-12, N2O, ClONO2, HCl, and HF will aid validation efforts for ILAS-II and SCIAMACHY, other atmospheric chemistry instruments, as well as enhance the interpretation of SAGE III observations.
The Esrange balloon and rocket launch facility near Kiruna, Sweden is the primary SOLVE-II balloon launch location due to its proximity to the latitude of SAGE III sunset occultations. Launches will be centered on the 3-4 week DC-8 deployment period in January/February 2003. However, launches may occur as early as late November 2002 and as late as April 2003 to maximize satellite comparison opportunities.
2.2.3 Ground-based Observations
During SOLVE-II, concurrent ground-based observations will also be made from Sweden, Norway, Finland, Germany, and Switzerland.
2.3 Geography
Kiruna, Sweden is located at 67.8 deg N latitude, 20.4 deg E longitude, about 200 km (125 miles) north of Arctic Circle. The town of Kiruna is approximately 500 m above sea level and has a population of about 25,000. It is the northern most municipality in Sweden, in the region known as Lapland. Wintertime conditions in and around Kiruna can be very severe, and during the coldest months, December, January and February, the average temperature is normally around -15 degrees Celsius (5 degrees Fahrenheit).
Kiruna was chosen as the deployment site for the SOLVE campaign for two reasons. First, the Arena Arctica facility at the Kiruna airport is a superb hangar and laboratory for operations during the arctic winter for the DC-8 and the European aircraft, and the hangar can easily house the many scientific instruments which will be flown during the mission. Second, Kiruna's extreme northern latitude is ideally located for measurements of the lower stratospheric polar vortex. Since Kiruna is at the edge of the winter polar vortex, the campaign aircraft can easily measure from the edge to the center of the vortex. Kiruna is also located near the climatological average coldest region in the Arctic. Proximity to the cold temperatures in this region is essential for the sampling of the PSCs during the winter period.
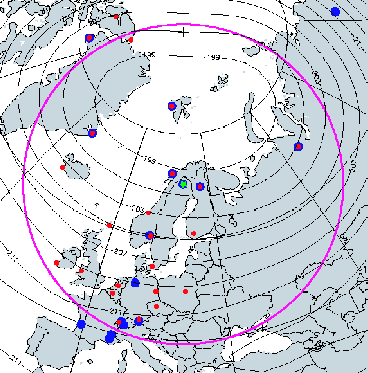
Figure 8 shows the SOLVE II operations region, with Kiruna, Sweden, noted at the center of the image with the green star. The magenta line indicates a distance of 2700 km from Kiruna. The blue dots indicate ground observation sites, while the red dots indicate ozonesonde sites. Also superimposed are 50-hPa January average temperature contours (1979-1999).
2.4 Forecasting
Meteorological forecasts of ground conditions and stratospheric fields will be required for directing DC-8 and balloon operations. The investigators will actively participate in mission development activities and in-field operations.
Information Sources
Newman, P. A., et al., An overview of the SOLVE/THESEO 2000 campaign, J. Geophys. Res., 107(0), XXXX, doi:10.1029/ 2001JD001303, 2002.
NASA Research Announcement, NRA-02-OES-02, April 1,2002.
SAGE III Ozone Loss and Validation Experiment (SOLVE) website.
Newman, P. A., SOLVE-II/VINTERSOL Science Goals and Mission Description, SOLVE-II Science Meeting, Dryden Flight Research Center, December 11, 2002.
Miller, C., SOLVE II Experimenter's Bulletin, September 13, 2002.