SAGE III Ozone Loss and Validation Experiment, SOLVE
A NASA DC-8, ER-2 and High Altitude Balloon Mission
1.0 SOLVE Science Overview
The SAGE III Ozone Loss and Validation Experiment (SOLVE) is a measurement campaign designed to examine the processes which control polar to mid-latitude stratospheric ozone levels.
1.1 Basic facts
1.1.1 What is ozone?
Ozone is an atmospheric gas which screens a harmful form of solar radiation known as ultraviolet radiation. About 90% of the ozone in our atmosphere is contained in the stratosphere (the region from about 30,000 feet to 180,000 feet above the Earth's surface between the tropopause and stratopause). Figure 1 shows a typical profile of ozone density versus altitude (yellow line). Ozone concentrations are greatest between about 50,000 and 100,000 feet, but these ozone concentrations are very small, typically comprising only a few molecules per million molecules of air (air is composed of about 78% nitrogen molecules and 21% oxygen molecules).
Figure 1. A typical vertical profile of ozone density in the midlatitudes of the northern hemisphere (units=Dobson Units/kilometer). The stratosphere lies between the tropopause and stratopause (marked in red). Superimposed on the figure are plots of UV radiation as a function of altitude for UVa (320-400 nm, cyan), UVb (280-320 nm, green), and UVc (200-280 nm, magenta). The width of the bar indicates the amount of energy as a function of altitude. The UVc energy decreases dramatically as ozone increases because of the strong absorption in the 200-280 nm wavelength band. The UVb is also strongly absorbed, with a small fraction reaching the surface. The UVa is only weakly absorbed by ozone, with some scattering of radiation near the surface.
Postscript file
1.1.2 UV effects and ozone
Absorption of harmful solar ultraviolet radiation is critical for our well being, since ultraviolet light can break the bonds of DNA molecules (the molecular carriers of our genetic coding), and thereby damage cells. While most plants and animals are able to either repair or destroy their damaged cells, on occasion, these damaged DNA molecules are not repaired, and can replicate, leading to dangerous forms of skin cancer in humans (basal, squamous, and melanoma). Fortunately, ozone strongly absorbs UV. Figure 1 also shows the amount of solar energy at various altitudes for 3 wavelength bands: UVc (200-280 nanometers, purple), UVb (280-320 nm, green), and UVa (320-400 nm, sky-blue). UVc is completely absorbed by small amounts of ozone and cannot penetrate to the surface. Note how the purple bar quickly diminishes as the solar energy penetrates to levels where ozone is found. UVb is also strongly absorbed, but some UVb can penetrate to the surface. If we reduced ozone by 1%, there would be about a 2% increase of surface UVb. Only about 1/2 of the UVa is absorbed or scattered. Diminished levels of ozone lead to increases of UVb and UVa radiation at the surface with a consequent adverse biological response.
1.1.3 Ozone production and loss
Ozone is produced by intense ultraviolet radiation in the upper stratosphere, wavelengths generally less than 240 nm. This radiation breaks apart an oxygen molecule into oxygen atoms and these atoms react with other oxygen molecules to form ozone molecules. This ozone production process is schematically shown in Figure 2.
Figure 2. Ozone is created when energetic UV light (wavelengths less than 240 nm, not visible), breaks apart (photolyzes) an oxygen molecule into two oxygen atoms. These O atoms react with other oxygen molecules (plus a third molecule, noted as M here) to form ozone.
Postscript file
The ozone molecule spends most of its life absorbing UV. Ozone is destroyed when it reacts with one of a variety of chemicals in the stratosphere such as chlorine, nitrogen, bromine or hydrogen. Figure 3 portrays the destruction of ozone in a homogeneous gas phase reaction.
Figure 3. Ozone is destroyed in a 3 step catalytic process. The first ozone molecule is photolyzed to form an O atom and and the first oxygen molecule. In step 2, the catalyst reacts with another ozone molecule to form XO and the second oxygen molecule. Finally, the XO molecule reacts with the O atom to form a third oxygen molecule, and to reform the original catalyst. The catalyst converts 2 oxone molecules into 3 oxygen molecules without being affected itself. A typical chlorine atom can destroy thousands of ozone molecules in this fashion.
Postscript file
The catalyst (X, the black molecule) in Figure 3 represents a chlorine, bromine, hydrogen, or nitrogen atom. Such reactions occur naturally, and the global ozone levels are approximately balanced between the solar production and chemical losses from these processes. Increasing the levels of chlorine and other pollutants in the stratosphere could increase the chemical loss process, and subsequently decrease the ozone levels.
1.1.4 Polar ozone science
The winter Arctic lower stratosphere is a strange environment for both chemistry and meteorology. During the winter, Arctic temperaturesat about 20 km can fall below -120 F (-83 C). In the Antarctic, the temperatures are even colder, occasionally falling below -136 F. Although the stratosphere is very very dry, these extremely cold temperatures cause clouds to form. These polar stratospheric clouds (PSCs) enable very interesting chemical reactions (known as heterogeneous chemical processes) that accelerate ozone loss. In fact, these heterogeneous reactions cause the Antarctic ozone hole. Scientists are increasingly worried that cooler temperature in the Arctic may lead to increased formation of PSCs, and thereby massive ozone losses. The ability to predict these losses requires detailed understanding of the chemistry, dynamics, and radiative properties of the Arctic stratosphere.
1.1.4.1 Antarctic and Arctic ozone levels
The Antarctic ozone hole is a region of extreme ozone loss that has been appearing annually since the 1970s. Ozone amounts over Antarctica drops dramatically (up to 50%) in the course of a few weeks during the August-September period. The ozone hole results from the increased amounts of chlorine and bromine in the stratosphere, combined with the peculiar meteorology and extreme cold of the southern hemisphere winter. Figure 4 shows October averages of satellite data for a series of early years, prior to the appearance of the ozone hole (top panels), and the last few years (bottom panels).
Figure 4. October averages of satellite column ozone as measured by Nimbus-4 BUV (1970, 72, 72), Nimbus-7 (1979), Meteor-3 (1994), SBUV (1996), and EP-TOMS (1997, 98). The color scale on the bottom indicates high ozone in red, and low ozone in the blues and purples.
Postscript file
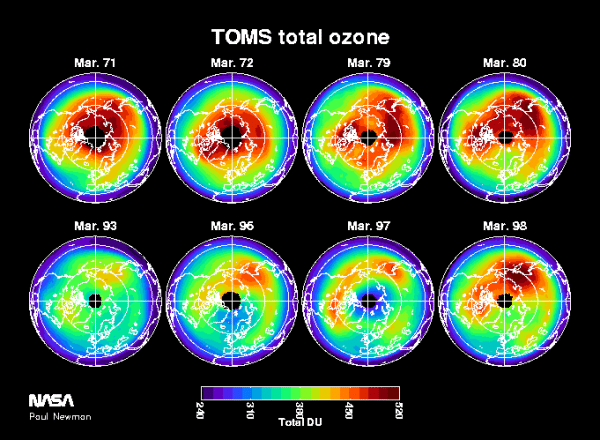
The growth of chlorine and bromine levels in the stratosphere has produced very large losses of ozone over the polar regions, producing this Antarctic ozone hole. During the 1990s, ozone levels are less than half of what was observed during the 1960s and 70s. Losses of a similar magnitude have been observed over the Arctic over a number of years. Figure 5 shows polar averaged Arctic and Antarctic ozone levels. Because of the different climates of the Arctic and Antarctic, the Arctic ozone levels are naturally higher, yet both polar regions have shown reduced ozone over the last decade.
Figure 5. March averages of total ozone as measured by Nimbus-4 BUV (1971, 72), Nimbus-7 (1979, 90, 93), NOAA-14 SBUV-2 (1996), and EP-TOMS (1997). Note the differences in color scales between Figures 4 and 5, northern ozone levels are naturally much higher than southern levels.
Postscript file
Using a number of satellites, the downward trends in both hemispheres are very apparent when polar cap averages are computed during the early spring. Figure 6 displays the polar averaged ozone in the Arctic during March (top line), and the Antarctic during October (bottom line). Ozone levels and temperatures in the Arctic are naturally higher than in the Antarctic. The downward trend of ozone is apparent in both the Arctic and Antarctic.
Figure 6. March averages of total ozone in the Arctic (top line) during March, and the Antarctic (bottom line) during October. The averages are computed in the polar region poleward of 63 degrees from a variety of satellites (see legend in bottom left corner).
Postscript file
The extremely low ozone years in the Arctic during the 1990's are characterized by colder than normal temperatures. The cold temperatures lead to an increase in the occurrence of polar stratospheric clouds (PSCs). The PSCs then lead to increased ozone loss via the heterogeneous reactions that occur on the surfaces of the PSCs as will be discussed in the following sections.
1.1.4.2 Polar Stratospheric Clouds
As the stratosphere cools to very cold temperatures over the Antarctic during the southern winter, polar stratospheric clouds (PSCs) form. Figure 7 shows a photograph of a PSC taken by Brian Toon near Iceland during the AASE I campaign in 1989. PSCs are particularly prevalent in the Northern Scandanavia region during the winter period. Kiruna is an optimal site for sampling PSCs , since the stratosphere above Kiruna is normally quite cold, and the local meteorology (mountain forced waves) creates even colder conditions, leading to PSC formation.
Figure 7. PSC observed during AASE I near Iceland (photo courtesy of O. B. Toon).
Postscript file
PSCs are principally responsible for the observed ozone losses in the polar region. These PSCs convert inorganic chlorine from reservoir species (HCl and ClONO2) to free radical form as in the Antarctic [see Brune et al., 1990; Toohey et al., 1993]. This conversion occurs via heterogeneous reactions on the surfaces of stratospheric particles and aerosols. Direct in-situ observations of PSCs are rather rare, having only occurred during a few flights of the ER-2 during the AAOE , AASE I, and ASHOE/MAESA campaigns.
1.1.4.3 Photochemical ozone loss
Arctic losses are related to these higher chlorine and bromine levels combined with colder temperatures which have begun to appear over the Arctic during the 1990s. As discussed, Figure 5 displays a series of March averages of total ozone over the Arctic. During 1997, temperatures in the Arctic were extremely cold, and ozone loss was quite large.
Substantial polar ozone loss requires 3 key ingredients. First, there must be significant levels of inorganic chlorine for conversion into radical forms. This condition has been true since the early 1980s as a result of pollution of the stratosphere by chlorofluorocarbons. Second, temperatures must be cold enough to form PSCs. As noted in the previous section, the temperature must fall below about 195 K for PSCs to form. This always happens in the Arctic by early December. Finally, the cold temperatures must persist late into the winter and early spring when the sun is fully risen over the Arctic.
Heterogeneous chemistry involves the conversion of the inorganic forms of chlorine (HCl, and ClONO2) into reactive forms occurs on the surfaces of PSCs. A simple cartoon of a very important heterogeneous reaction is shown in Figure 8. First, HCl and ClONO2 stick to the surface of our PSC (frame 1), the HCl and ClONO2 react on the surface of the PSC to form Cl2 and HNO3 (frame 2), the Cl2 is outgassed from the PSC while the HNO3 remains on the PSC (frame 3), and finally the Cl2 is photolyzed by sunlight (frame 4) with the two Cl atoms initializing the catalytic loss of ozone.
Figure 8. Cartoon illustration of the conversion of reservoir species of chlorine (HCl and ClONO2) into reactive chlorine that can destroy ozone.
Postscript file
The reaction shown in Figure 8 is only 1 of a series of heterogeneous reactions that are recognized for their importance in stratospheric chemical processes. Other reactions include:
1. N2O5 + H2O -> HNO3
2. ClONO2 + H2O -> HOCl + HNO3
3. ClONO2 + HCl -> Cl2 + HNO3
4. BrONO2 + H2O -> HOBr + HNO3
5. HOCl + HCl -> Cl2 + H2O
6. HOBr + HCl -> BrCl + H2O
Reactions 2, 3, 5, and 6 repartition the Cly towards ClO, while reactions 1-4 shift NOx into HNO3, suppressing the NO2 that will deactivate the ClO. These reactions depend on both the temperatures, and the type of the particle on which the reaction takes place. Figure 9 shows the sensitivity of the reactions (sticking coefficients) to temperature for various reactions and particle types. /
Figure 9. Sticking coefficients versus temperature from a sample of laboratory measurements on liquid sulfate, SAT, and NAT. Note that the y-axis is logarithmic (each major tick mark is a power of 10), hence the liquid sulfate reaction (top left panel) increase by a factor of 10,000 as the temperature decreases from 204 K to 190 K.
Postscript file
The extreme temperature sensitivity of these heterogeneous reactions has tremendous consequences for Arctic polar ozone. For example, if the polar region cooled from 200 K to 195 K, the rection of HCl and ClONO2 on a NAT particle (bottom right of Figure 9) increases 10 fold. Hence, a small cooling in the Arctic stratosphere or a shift to colder temperatures in late-winter will lead to much larger ozone loss.
1.1.4.4 Dynamics and transport
The polar lower stratosphere becomes extremely cold during winter. Figure 10 shows the average temperatures from the surface to about 157,000 feet (the NASA ER-2 can reach altitudes of about 70,000 feet or 20 km). The polar night jet acts to contain the polar air during mid-winter. This polar air mass is known as the polar vortex. As viewed from space above the North Pole, this appears as a counterclockwise swirl of air. Because of this containment, this polar votex air has a distinctive chemical composition as compared to air in the mid-latitudes. This vortex containment makes the polar vortex a "containment vessel" for the chemical ozone loss that takes place during the winter and spring. Because the cold temperatures are found in this high latitude region, and because of the "containment", it is necessary to make measurements from the high latitude site of Kiruna, Sweden.
Figure 10. Twenty year average of January longitudinally averaged temperatures (in color), and winds (white contours). Cold temperatures (in purple) are found poleward of 60N in the lower stratosphere, and over the tropics at about 16 km (50,000 feet). The polar vortex is the region poleward of the polar night jet (indicated in the figure) above the tropopause. The tropopause is the thick black line.
Postscript file
The air found in the polar vortex principally originates from higher stratospheric altitudes. Figure 11 shows this descent process at ER-2 flight altitudes from October to March. The color maps at the top show a quantity known as potential vorticity or PV. PV simply highlights the geographic origin of the air. In these images, polar air is represented by the reds and orange colors, while tropical air is purple. The mid-latitude air is blue to yellow in color. The black dots represent idealized balloons (i.e., air parcel trajectories) that follow the motion of air.
Figure 11. Potential vorticity images for October 1, 1994 and March 1, 1995 on the 500 K isentropic surface (approximately 20 km). The blue line in the top images shows the approximate position of the vortex edge. The regular grid of small dots superimposed on the high PV values in the top right image (March 1) represent the initial positions of back trajectory parcels initialized inside the polar vortex. The dark points on the October 1 image are the final positions (starting points) of this air that was found inside the vortex on March 1. The bottom plot shows the pressure altitudes of these parcels on October 1 (white dots) and March 1 (blue dots). The air has descended about 150 K (4-5 km) over this 150 day period.
Postscript file
The air parcels (black dots) on October 1 (top left panel of Figure 11) are initially scattered over the northern latitudes at an altitude of about 27 km (90,000 feet), with a few air parcels in the mid-latitudes. By early March, these air parcels have concentrated inside the polar vortex and descended to about 21 km (70,000 feet). The air generally moves poleward and downward over the course of the winter period. Since ozone values increase with altitude, this poleward and downward motion increases the column ozone amount. Therefore, the average transport of air during the winter will act to increase the column ozone over the course of winter.
1.1.5 Ozone and public policy
Reported ozone losses and predictions of ozone loss have heightened our concern with increasing UV levels. Such concerns have led to the international treaties such as the Montreal Protocol , which have regulated the production of certain gases which harm the ozone layer. Further research and measurements has begun to provide insight into how other atmospheric processes (such as greenhouse warming) can effect ozone. This further research in necessary to refine our understanding of the stratosphere, leading to well based policy decisions.
1.2 SOLVE science goals
SOLVE is an Arctic mission which will be conducted over the course of the 1999-2000 winter. SOLVE will employ aircraft, satellites, and balloon to examine the processes which control polar to mid-latitude stratospheric ozone levels. The mission will also acquire correlative measurements needed to validate the Stratospheric Aerosol and Gas Experiment (SAGE) III satellite mission and will use these satellite measurements to help quantitatively assess high latitude ozone loss.
1.2.1 Ozone observations
1.2.1.1 Observed changes in high latitude winter ozone
The growth of chlorine and bromine levels in the stratosphere has produced very large losses of ozone over the polar regions, producing the famous Antarctic ozone hole (see section 1.1.4.1). During the 1990s. Losses of a similar magnitude have been observed over the Arctic over a number of years. Figure 6 shows polar averaged Arctic and Antarctic ozone levels. Because of the different climates of the Arctic and Antarctic, the Arctic ozone levels are naturally higher, yet both polar regions have shown reduced ozone over the last decade. The principal question for SOLVE is to explain these losses with detailed measurements of the chemistry, dynamics and photolytic properties of the stratosphere.
1.2.1.2 Polar ozone loss during mid winter.
Results from the 1995 DC-8 Tropical Ozone Transport Experiment and Vortex Ozone Transport Experiment, TOTE/VOTE along with modeling studies suggest that models cannot currently account for the amount of ozone lost during the winter [Hansen et al., 1997; A. Douglass, private communication]. This discrepancy has also been reported in comparisons with data taken during recent European campaigns (Hansen et al., 1997; Bregman et al., 1997). We apparently have a poor understanding of the balance of ozone production, loss and transport in the lower stratosphere in the early winter period where chlorine chemistry is not very active.
1.2.1.3 Uncertainties in the calculations of ozone losses.
Various techniques are employed to calculate ozone loss in the northern polar region. Newman et al. [1997] used TOMS total ozone data to calculate a trend of ozone that showed that levels in the 1990's were about 100 DU lower than levels observed in the 1970's (Figure 6). Muller et al. [1997] used HALOE observations to estimate a 70-80 DU loss of ozone during March 1997 using methane as a conservative tracer of atmospheric motion. Von der Gathen et al. [1995] has combined ozonesondes and trajectories to estimate a 38% loss of ozone at the 475K isentropic surface during the January-February 1992 period. Rex et al. [1997] used the same technique to calculate a 64% loss from 20 January 1995 to 9 April 1996.
SOLVE will validate and assess uncertainties associated with these ozone loss techniques. We will quantitatively determine relative contributions to low ozone levels from interannual variations in ozone transport, and ozone photochemistry. As halogen levels decrease in the stratosphere, a clear understanding of the uncertainties associated with the loss calculations is necessary to determine statistical significance.
1.2.2 Polar stratospheric clouds
1.2.2.1 Properties of Polar stratospheric clouds
One of the major issues concerning polar ozone loss is to understand the composition of PSCs. Reaction rates vary depending upon whether the aerosols are solids or liquids (see Figure 9). They also vary with cloud exposure, frequency, and conditions of cloud formation. At present it is thought that many of the clouds are supercooled nitric acid/sulfuric acid/water solutions. However, DC-8 lidar data from AASE Iindicate that the aerosols were solids more that half the time on every flight on which PSCs were observed (Browell et al., 1990).
1.2.2.2 Conversion of nitrogen and chlorine reservoir species.
The critical period for ozone loss occurs as the sun begins to return to the Arctic region during the January-April period. As air inside the polar vortex cools during November and December (section 1.1.4), particles will form. Heterogeneous reactions (see section 1.1.4.3) will occur as this air passes through the cold temperature regions (i.e., the air is processed by heterogenous reactions) This initial processing typically occurs in December. Temperatures typically rise above the nitric acid tri-hydrate saturation temperature in February. Because air can still be processed on cold sulfate aerosols, processing becomes insignificant when temperatures rise above about 205 K in spring. Reaction probabilities on cold sulfate aerosols increase by nearly four orders of magnitude as the temperature decreases from 205 to 190K (see Figure 9). Hence, the February to March period is critical for understanding ozone loss, since most of the inorganic chlorine has been activated into reactive form by these particles, and the sun has steadily risen over the Arctic providing the energy flux necessary for fueling these ozone loss catalytic reactions.
1.2.2.3 Denitrification.
Denitrification is the process of removing nitrogen compounds from the stratosphere. Since nitrogen compounds such as HNO3 and ClONO2 play key roles in shutting down catalytic ozone loss by ClO, denitrification may strongly enhance ozone loss by delaying this shut down.
Denitrification in the Arctic is much less extensive than over Antarctica. Because Arctic temperatures are distinctly warmer than Antarctic during winter, the formation of large particles than can settle out of the stratosphere should be substantially smaller. Further, the Arctic polar vortex is warmer and weaker in late winter, and usually breaks up significantly earlier than the Antarctic polar vortex. Temperatures cold enough to form ice particles (~188 K) are infrequent in the northern hemisphere. In the case of temperatures, while Antarctic lower stratospheric temperatures usually drop well below the frost point in mid-winter, the Arctic vortex has only a few days in which temperatures fall below the frost point (at least on synoptic scales). Sampling of such extremely cold events will add a key piece of evidence to our understanding of denitrification.
The degree of ozone loss is extremely sensitive to the timing of the disappearance of cold temperatures and polar vortex breakup. Temperatures fall below 200 K beginning in mid-November and have typically risen above 200 K in about mid-March. Sampling of air that is in the temperature range 195-205 K should yield evidence of processing of air by cold aerosols during the late-winter breakup period.
Recent work by Carslaw et al. [1998] analyzed the impact of mountain forced gravity waves on the temperatures of the lower stratosphere. Figure 12 (adapted from Carslaw et al., [1997]) displays backscatter ratios for PSC's observed over northern Scandanavia.
Figure 12. A mountain wave PSC observed by the airborne OLEX lidar
over northern Scandinavia. Courtesy of M. Wirth, DLR, Germany. For more details
see Carslaw et al. (1998)
Postscript file
Because there are large vertical excursions of air within these waves, there are also large temperature excursions as air parcels move through these waves. Such events can have a significant impact on chlorine activation in the polar region, since the time scales for activation are short while chlorine deactivation time scales are long, the flow speeds through these mountain wave events are fast, and there are a number of mountain ranges in the northern hemisphere which can spawn such waves. The spatial and temporal evolution of such waves are not observable by the conventional synoptic network and satellite measurement techniques. Observations of such waves by aircraft will significantly improve our understanding of mesoscale features on the chemistry of the polar vortex and mid-latitudes.
1.2.3 Photochemical processes
1.2.3.1 Chlorine activation
Chlorine activation involves a number of science questions. First, what is the seasonal evolution of the particles and how does this evolution impact reaction probabilities over the winter season? Second, what is the chemical evolution of the nitrogen and chlorine budgets over the course of the winter due to repeated activation events? In particular, the impact of mesoscale temperature perturbations that result from mountain waves (see Figure 12) needs to be considered in addition to the activation events that result from synoptic scale systems. Additional questions involve the details of denitrification processes that extract reactive nitrogen from the lower stratosphere.
Data presented by Avallone et al. (1993) showed that ClO in the lower stratosphere was enhanced relative to the concentrations calculated from models that include heterogeneous conversion of N2O5 to nitric acid on sulfate aerosol (reaction 1 in section 1.1.4.3). Ozone loss rates from measured ClO were much larger than predicted. Avallone et al. speculated that ClONO2 is a much larger fraction of the inorganic chlorine budget than models predict. Since Avallone et al. (1993), lab measurements showed that heterogeneous reactions of bromine compounds (e.g., BrONO2, HOBr) are considerably faster than their chlorine counterparts. It is possible that such reactions convert some HCl into ClONO2. Because HCl is the dominant reservoir of inorganic chlorine in the lowermost stratosphere and active chlorine is more easily released from ClONO2 than from HCl, even a small conversion of HCl to ClONO2 can result in significant ClO enhancement.
Solomon et al. (1997) proposed that inorganic chlorine can also be activated in cirrus clouds at the lowest stratospheric altitudes. The resulting increases of ClO could be responsible for significant ozone losses observed by SAGE II. Unfortunately, there are few measurements of ClO in cirrus, and these are ambiguous (Borrmann et al., 1997). Solomon et al. (1997) predicts that heterogeneous reactions of ClONO2 and HCl can enhance ClO mixing ratios to 10 ppt in summer and 50 ppt or higher in winter near the tropopause.
1.2.3.2 Chlorine deactivation and ozone loss
The processes in the ozone loss cycles are:
ClO + ClO -> ClOOCl
ClOOCl + light -> Cl + ClOO
ClOO + M -> Cl + O2 + M
2 x (Cl + O3 -> ClO + O2)
Net: 2 O3 -> 3 O2
And
ClO + BrO -> Cl + Br + O2
Cl + O3 -> ClO + O2
Br + O3 -> BrO + O2
Net: 2 O3 -> 3 O2
Our current understanding of the termination of this halogen catalyzed ozone loss depends on the reintroduction of NO2into the gas phase where it reacts with ClO to form the relatively long-lived ClONO2. The NO2 is formed by the photolysis of nitric acid (HNO3 + UV light -> NO2 + OH).
The key chemistry questions involve: whether NO2 is the principal gas for deactivating ClO; how do the reservoir species (ClONO2 and HCl) evolve following ClO deactivation; whether the formation of ClOOCl a principal part of the loss mechanism; and what are the relative contributions among catalytic loss cycles to the overall ozone loss in the winter to spring period?
The total loss of ozone is determined by these catalytic reaction chain's competition with the warming temperatures during spring. If the cold temperatures persist into late March, the chlorine is maintained at high levels and the nitrogen continues to be sequestered in HNO3. With 1) high reactive chlorine levels, 2) sequestered nitrogen, and 3) solar visible and UV in the northern polar region, the ozone loss rates will be extremely large. Hence, the details of the ClO deactivation is critical to an accurate calculation of net ozone loss.
1.2.4 Transport and dynamics
Understanding and measuring the initial state of air comprising the Arctic stratosphere is important for interpreting the subsequent cold temperature chemistry and ozone loss. In order to understand the final state of the polar vortex in late spring, it is critical to measure the initial states for O3, the nitrogen (NOy) and halogen (Cly) compounds, since these constituents will interact with aerosols, and the HNO3 and H2O vapors that condense to form PSCs. Finally, remote observations can define initial profiles of total reactive chlorine (Cly) and nitrogen (NOy), as well as H2O, CH4, HNO3, HCl, ClNO3, and O3 within the vortex. These initial profiles are crucial to understanding the conditions of the vortex during the late winter period when detailed process studies can be conducted by the ER-2 and DC-8.
Observations of the initial state of the Arctic stratosphere will allow for better understanding of the role of transport on the evolution of Arctic O3 during a typical winter, as well as more reliable model predictions of the sensitivity of vortex composition to exhaust from (current and future) subsonic and (future) supersonic aircraft.
The exchange of air between the polar vortex and the mid-latitudes is a key component of the polar ozone budget. The carrying of low ozone air from the polar vortex into mid-latitudes can will reduce mid-latitude ozone levels. In addition, while mid-latitude air is not easily carried into the polar vortex, occasionally under disturbed stratospheric weather conditions, mid-latitude air can be entrained into the vortex. Such an intrusion occurred in late January 1992 (see Plumb et al., 1993). Figure 13 displays an image of this disturbed vortex (red colors indicates polar air, purple is tropical, light-blue and green air is mid-latitude).
Figure 13. Potential vorticity image for January 24, 1992, on the 450 K isentropic surface (approximately 18 km). The red air is polar material, while purple shows tropical.
Postscript file
This situation shows an extreme example of polar air being carried into the mid-latitudes, while mid-latitude air is stirred into the middle of the polar vortex. The frequency and quantitative volume exchange is key to understanding the evolution of polar ozone levels.
1.2.5 SAGE III Validation
Because of the importance of stratospheric ozone levels, satellite and ground sites constantly measure the levels of ozone in our atmosphere. The United States maintains 3 basic systems for measuring ozone from space: the Stratospheric Aerosol and Gas Experiment (SAGE), the Solar Backscatter UltraViolet Experiment (SBUV), and the Total Ozone Mapping Spectrometer experiment (TOMS). These satellite instruments provide near real time monitoring of ozone in our atmosphere.
The SAGE instruments uses a technique known as solar occultation to measure ozone. Ozone absorbs solar radiation as it passes through the atmosphere. The SAGE instrument views the sun as the satellite orbits the earth. During its orbit, the SAGE instrument sees numerous sunsets. As the edge of the Earth begins to block the sun's radiation, the SAGE instrument peers through the atmosphere at the sun. Since the solar radiation is absorbed, the amount of absorption depends on the ozone amount.
The veracity of the satellite measurements is tested by comparisons to other measurement techniques. These comparisons are necessary for proving the quality of the observations for the necessary trend studies that are used to set public policy. Numerous sites in the Northern polar latitudes measure ozone by a variety of techniques, including lasers, balloons, microwave measurements, and UV measurements. In addition, aircraft measurements can be employed as validation tools.
2.0 Science Implementation
The science implementation is principally derived from the science questions that were put together by a NASA working group and the SOLVE science team. Platforms, the deployment site, and schedules have been optimized (within $ constraints) to answer the SOLVE science questions. The SOLVE mission will be staged over the course of the northern winter of 1999-2000, and is based on aircraft, balloons, satellites, and ground instruments. The aircraft and balloons will be flown from Kiruna, Sweden.
2.1 Platforms
2.1.1 ER-2
The NASA ER-2 has been successfully deployed as part of a long series of stratospheric missions, including STEP, AAOE, AASE I, AASE II, SPADE, ASHOE/MAESA, STRAT, and POLARIS. The ER-2 can carry a 2500 lb. payload to an altitude over 65,000 feet. The ER-2 can fly a distance out and back of approximately 2400 km. This allows the ER-2 to make measurements as far north as the North Pole on an extended sortie. Because the ER-2 flys in the stratosphere where polar stratospheric clouds form, it is an ideal platform for direct sampling (in-situ) of stratospheric chemistry and dynamics.
2.1.2 DC-8
The NASA DC-8 is a flying laboratory that has been coverted from a commercial airlinger. The DC-8 has also been successfully deployed as part of a long series of stratospheric missions, including AAOE, AASE I, AASE II, SONEX, and VOTE/TOTE. The DC-8 can carry a 30,000 lb. payload to an altitude over 35,000 feet. The DC-8 can fly a distance out and back of approximately 4000 km. This allows the DC-8 to make measurements over a substantial fraction of the northern hemisphere. Because of it's heavy lift and long range capability, the DC-8 can carry heavy remote sampling instruments such as lidars . These remote sampling instruments can make measurements to extremely high altitudes, and provide a unique measurement complement to the ER-2 in-situ observations.
2.1.3 Balloons
Balloons provide an important complement to the ER-2 in-situ sampling and the remote sampling of the DC-8, since the balloons can carry instruments up to altitudes over 100,000 feet. As part of both the STRAT and POLARIS missions, the OMS platforms have carried complementary instruments to the ER-2 payload.
Balloon-borne remote sensing observations provide an alternate validation means for the SAGE III satellite instrument. The balloon-borne observations of O3 and H2O, which have been compared extensively to in situ data and have served as correlative measures for a number of instruments aboard The Upper Atmosphere Research Satellite (UARS), will be used in a similar capacity for SAGE III validation. In situ observations of O3 and H2O will also be used for such validation efforts. The remotely sensed balloon-borne observations of O3 and H2O can serve as a bridge between the in situ and SAGE III observations because both SAGE III and the balloon FTIR are solar occultation instruments with comparable vertical and horizontal resolution. Measurements of NO2 by an FTIR instrument will be used for validation of SAGE III measurements of this species; similar viewing geometries are particularly helpful owing to the strong dependence of concentrations of NO2 on solar zenith angle. In situ measurements of aerosol surface area will provide validation for SAGE III estimates of this parameter. The balloon-borne instruments will not obtain observations of NO3 and OClO, two gases measured by SAGE III. However, measurements of vertical profiles of every important gas in the NOy family other than NO3 (e.g., NO, NO2, HNO4, N2O5, ClNO3, and HNO3) as well as O3 by an FTIR solar occultation instrument will, together with photochemical models, provide useful constraints for the interpretation of SAGE III measurements of NO3. In a similar manner, the balloon-borne observations of ClO, HCl, and O3 by a submillimeter wave emission instrument will be particularly useful for guiding the interpretation of SAGE III measurements of OClO.
2.1.4 SAGE III and other satellite instruments
Satellite instruments play a key role in the monitoring of the Earth's ozone layer. As discussed in section 1.2.5, SAGE III validation is a principal goal of SOLVE. However, SAGE III data provide a key element for interpreting the evolution of polar stratospheric ozone over the course of the winter. Figure 14 displays the SAGE III observations patterns over the course of the winter of 1999-2000.
Figure 14. Sample time-latitude coverage of METEOR/SAGE III measurements. Local satellite sunset (sunrise) occultation events are indicated by solid (dashed) lines. Moonset (moonrise) occultation events are indicated by solid (open) circles.
Postscript file
The SAGE III sunset occultations are all concentrated in the northern polar region, making this satellite ideal for monitoring the evolution of polar ozone levels.
2.2 Science plan implementation
The instruments, measurements, and overall payloads for the aircraft and balloons are derived from the science questions. The ER-2 payload consists of 18 instruments. The DC-8 payload consists of 15 instruments. Their N sites taking ground based measurements, and their are X theory teams.
2.1 Deployment site
Kiruna, Sweden was chosen as the deployment site for SOLVE for 2 reasons. First, the Arena Arctica facility at the Kiruna airport is a superb hanger for the ER-2 and DC-8 operations. Second, Kiruna is ideally sited for measurements of the lower stratospheric polar vortex. Figure 15 displays a 20 year average of the polar vortex using potential vorticity at about 20 km (the approximate flight altitude of the ER-2).
Figure 15. A 20 year average (1979-1998) of January potential vorticity at 480 K (approximately 20 km) illustrating the average position of the Arctic polar vortex. Red colors identify the region of high PV (the vortex), while green identifies mid-latitude air. The location of Kiruna, Sweden is noted by the star, and a 2000 km circle is drawn around Kiruna, illustrating the capability of both the ER-2 and DC-8 to sample this region.
Postscript file
On average, Kiruna is located at the edge of the polar vortex. On many days during January, Kiruna is located well inside the polar vortex.
In addition to its proximity to the polar vortex, Kiruna is also located near the climatological average coldest region in the Arctic. Figure 16 shows a 20 year average of temperatures from 1979 to 1998.
Figure 16. A 20 year average (1979-1998) of January temperature at 480 K (approximately 20 km) illustrating the average position of the cold temperatures within the Arctic polar vortex. The location of Kiruna, Sweden is noted by the star, and a 2000 km circle is drawn around Kiruna, illustrating the capability of both the ER-2 and DC-8 to sample this region..
Postscript file
On average, the coldest point in the Arctic lower stratosphere is located over Spitzbergen Island, a short flight from Kiruna. In fact, because of the waves forced by mountains near Kiruna, layers of extremely cold temperatures can be found directly above Kiruna. Proximity to cold temperatures is absolutely necessary for the sampling of PSCs during this winter period.
2.3.2 Deployment schedule
The overall deployment schedule is tailored to sample the Arctic region during the principal climatological periods of the winter. The first part of the SOLVE campaign begins in November with launches of the OMS in-situ and remote payloads just prior to the appearance of temperatures that are below 195 K, such that we obtain samples of the polar vortex just prior to the first appearance of PSCs. The overall deployment schedule is shown in Figure 17 with the superimposed temperatures.
Figure 17. A longitudinally (zonal) average of temperature taken from the October 1995 to April 1996 period. The SOLVE deployments are superimposed for the DC-8 (red), ER-2 (blue), and balloons (crossed circles). Temperatures below 195 K (purple) normally appear in early December.
Postscript file
The DC-8 begins its first deployment on December 1, 1999, immediately following the OMS flights. This first phase of the campaign will define the initial conditions of the polar vortex.
The second phase of the campaign starts in mid-January with a launch of the remote sensing OMS payload. The ER-2 and DC-8 both begin measurements on January 15. This phase of the campaign is tied to the period when PSCs are most probable in the northern polar region. During this phase, the aircraft will actively attempt to sample the cold regions and PSCs.
The final phase of SOLVE begins in late-February with a launch of the in-situ OMS payload. Again, the ER-2 and DC-8 will conduct 3-week and a 2-week deployments, respectively. These deployments are tied to the period when ozone loss is largest.
3.0 Summary
The SOLVE campaign designed to examine the processes which control polar to mid-latitude stratospheric ozone levels. The mission will be staged during the 1999-2000 northern winter from Kiruna, Sweden. The SOLVE campaign will employ the NASA ER-2, NASA DC-8, the OMS in-situ and remote sensing balloon payloads, ground station observations, and an extensive theory team. The results of SOLVE will be both expand our understanding of polar ozone processes, and will provide greater confidence in our current ozone monitoring capabilities. This knowledge provides the basis for setting sound public policies which will help to preserve the Earth's ozone layer.
Last Updated: 1999-03-02
Author: Dr. Paul A. Newman (NASA/GSFC, Code 916) (newman@notus.gsfc.nasa.gov)